CH3COOH NaHCO3 ----- CH3COONa H2CO3 Carbonic acid decomposes into carbon dioxide and water. Baking soda and water reaction equation.

Nahco3 Hc2h3o2 Baking Soda And Vinegar Youtube
2 out of 5-year rule rental property.

. 2 CH3COOH Na2CO3 ---- 2 CH3COONa H2O CO2. How much 10 M sodium bicarbonate solution in mL is needed to stoichiometrically react with 25 mL of 45 acetic acid. With s solid l liquid g gas aq aqueous or in water solution.
This reaction is called an exothermic reaction. Check the balance Sodium carbonate react with acetic acid to produce sodium acetate and sodium bicarbonate. Type of Chemical Reaction.
See answer 1 Best Answer. Click to see full answer. Sodium hydrogen carbonate Acetic acid Sodium acetate Carbon dioxide Water.
Write the balanced chemical equation for the reaction of sodium bicarbonate NaHCO3 and acetic acid HC H3O2. CHO NaHCo NaCHO H2O CO2. Acetic acid Sodium Carbonate ---- Sodium Acetate Water Carbon Dioxide.
NaHCO 3 HC 2 H 3 O 2 NaC 2 H 3 O 2 H 2 O CO 2. Baking soda vinegar carbon dioxide water sodium acetate NaHCO 3 aq CH 3 COOH aq CO 2 g H 2 O l CH 3 COONa aq Looking closely at this equation examine whether it is balanced or not. This is the balanced chemical equation for a reaction between acetic acid in vinegar and sodium bicarbonate in baking soda.
C7H6O3s C4H6O3aq C9H8O4s Chemistry. In this reaction we have NaHCO3 baking soda reacting with an aqueous solution of HC2H3O2. Write balanced chemical equations for all transferals of the compounds from organic to aqueous phases and for all precipitation reactions.
Sodium bicarbonate and acetic acid reacts to carbon dioxide water and sodium acetate. How many Hydrogen atoms are in the reactants___5_____ In the products___5_____. NaHCO 3 HC 2 H 3 O 2 NaC 2 H 3 O 2 CO 2 H 2 O.
When dry baking soda doesnt decompose very quickly although it does have a. Eventually all of the solid dissolved and reacted producing a new liquid solution. Also Know what is the balanced equation for sodium bicarbonate and acetic acid.
Acetic acid reacts with sodium bicarbonate to form sodium acetate and carbonic acid. Similarly what happens when you mix. The Balanced Equation.
Molecular weight of sodium bicarbonate 84007 gmol molecular weight of acetic acid 60052 gmol. The solution is red in color therefore the salt NaC2H3O2 in the solution is basic. Baking soda and water reaction equation.
The reaction between baking soda and vinegar actually occurs in two steps but the overall process can be summarized by the following word equation. Reaction with symbols NaHCO_3 CH_3COOH - CH_3COO Na CO_2 H_2O CH_3COONa - Sodium ethanoate. Na 2 CO 3 CH 3 COOH CH 3 COONa NaHCO 3.
Acetic acid is a weak acid with the formula CH3COOH the Ka for acetic acid is 176 x 10-5. CH 3 COOH NaHCO 3----- CH 3 COONa H 2 O CO 2. The balanced equation for the decomposition of sodium bicarbonate into sodium carbonate carbon dioxide and water is.
Examine the products you wrote in 2. Another common way to write this reaction is. The balance ionic equation for the reaction is.
The above reaction while technically correct does not account for the dissociation of the sodium acetate in water. The reaction is. Sodium bicarbonate and acetic acid react to form sodium acetate water and carbon dioxide gas.
Best sushi marlborough ma. During the reaction a solid and liquid have been chemically reacted to form a gas and a liquid. See the explanation Sodium bicarbonate - NaHCO_3 Acetic acid - CH_3COOH Reaction in words Sodium bicarbonate Acetic acid - Sodium ethanoate Carbon dioxide Water.
H 2 CO 3----- H 2 O CO 2 So the net ionic reaction is. In aqueous solution acetic acid partially dissociates according to the following reaction. What is the balanced equation for the neutralization of lactic acid using Sodium bicarbonate.
2 NaHCO3 s Na2CO3 s CO2 g H2O g Like most chemical reactions the rate of the reaction depends on temperature. Note that the ions for NaHCO3 are Na HCO3 and the ions for HC2H3O2 are H C2H302 NaHCO3 HC2H302 NaC2H302 H2O CO2 double displacement and decomposition What type of reaction did you write. Isner john vs pospisil prediction.
NaHCO3 aq HC2H3O2 aq CO2 g H2O l NaC2H3O2 aq The formation of yellow bubbles indicates an acidic gas is formed H2CO3 this comes from the interaction of CO2 H2O H2CO3. NaHCO3 HC2H302 NaC2H302 H2O CO2 or sodium bicarbonate plus acetic acid equals sodium Acetate plus. Molar mass of acetic acid CH3COOH is 60 gmol molar mass of sodium bicarbonate Na2 HCO3 is 84 gmol the rxn is Na2 HCO3 CH3COOH CH3COONa H2O CO2 We know At 1 atm pressure and 273 K temperature volume occupied by CO2 224 litre But this occ.
What is the balanced equation for sodium bicarbonate. The reaction can be explained with the equation of. For this reaction we have a chemical reaction.
CH3COOH CH3COO- H Use the Ka equation. The balanced chemical equation is.

How To Balance Nahco3 Hc2h3o2 Nac2h3o2 Co2 H2o Breslyn Org

Multimedia Controlling The Amount Of Products In A Chemical Reaction Chapter 6 Lesson 2 Middle School Chemistry

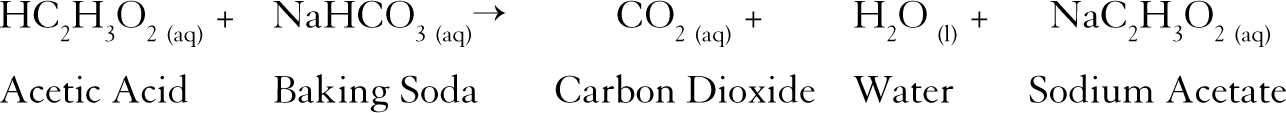
0 Comments